Department of research and modern medical articles
Introduction of Chedid drug for the treatment of local hair loss (alopecia areata) and regional hair loss
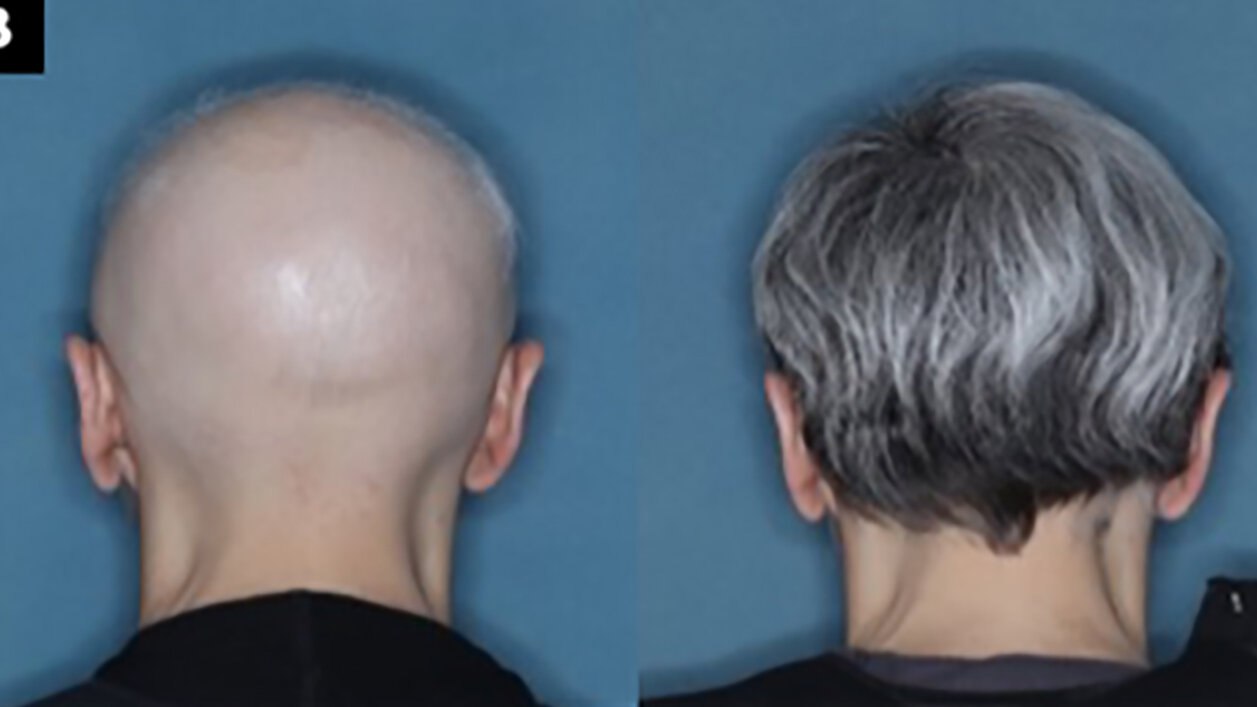
This drug called baricitinib has been approved by the FDA and is prescribed for hair regrowth. This drug, which is also prescribed for the treatment of rheumatic disorders, prevents the destruction of hair follicles by inhibiting the immune system. The side effects of the drug include acne and urinary infections. This drug is currently sold in the market by three companies, Ely Lilly, Pfizer, and Concert. It is said that based on field research conducted by Lilly Company on 1,200 patients, it was observed that forty percent of the people who used this drug After thirty-six weeks, they observed their hair growth, and after one year, half of the patients diagnosed with alopecia areata saw regrowth of hair.
This drug is also prescribed for rheumatic diseases.
https://www-nytimes-com.cdn.ampproject.org/v/s/www.nytimes.com/2022/06/13/health/alopecia-drug-approved-fda.amp.html?amp_gsa=1&_js_v= a9&usqp=mq331AQKKAFQArABIIACaw%3D%3D#amp_tf=From%20%251%24s&aoh=16553190636854&referrer=https%3A%2F%2Fwww.google.com&share=https%3A%2F%2Fwww.nytimes.com%2F2022%2F06%2F13% 2Fhealth%2Falopecia-drug-approved-fda.html
This post is written by Drmoghimii